双碱法脱硫 / Dual-alkali desulfurization
1、钠钙双碱法脱硫简介
钠-钙双碱法[NaOH或Na2CO3/Ca(OH)2]脱硫工艺,是在石灰石-石膏法的基础上结合钠碱法发展起来的一种目前国内成熟的脱硫工艺。其是利用钠盐易溶于水来吸收烟气中的SO2, 然后利用石灰粉再生脱硫液。由于整个反应过程是液气相之间进行, 吸收速率高,液气比低,吸收剂利用率高,投资费用省,运行成本低。脱硫反应过程为烟气与脱硫吸收塔内若干层数十支喷嘴喷出的细微雾化液滴进行充分汽液混合接触,使烟气中SO2被脱硫液充分吸收、反应,达到脱尘除SO2的目的,经脱硫洗涤后的烟气再经脱硫吸收塔顶部除雾器脱水后进入烟囱排入大气。脱硫循环液经塔底管道流入沉淀池,上清液经上部溢出进入反应再生池,在池内与石灰乳液进行再生反应,再生液流入泵前循环槽补入Na2CO3,由泵打入脱硫塔顶脱除SO2循环使用。脱硫产物经氧化最终是石膏浆CaSO4。
I.Introduction of sodium-calcium dual-alkali desulfurization.
Sodium-calcium dual-alkali [NaOH or Na2CO3/Ca (OH)2] desulfurization process is currently a mature domestic desulfurization process developed on the basis of limestone – gypsum methods combined with sodium-calcium method. Soluble sodium salt is used to absorb SO2 in flue gas, and then lime powder is used to form desulfurization solution. As the entire reaction process is carried out between liquid and gas, it has high absorption rate, low liquid gas ratio, high absorbent utilization rate, low investment cost and low operation cost. The desulfurization process: flue gas has gas-liquid contact with atomized liquid droplets sprayed from dozens of nozzles in desulfurization absorption tower; SO2 in flue gas is fully absorbed by and reacts with desulfurization solution to achieve the objective of SO2 removal. The flue gas after desulfurization is dehydrated by mist eliminator on top of absorption tower then is discharged from chimneys. Desulfurization cycle liquid flow through pipes at the bottom of the column into sedimentation tank; supernatant fluid flow into reaction and regeneration tank from the top and has regeneration reaction with milk of lime; acetate solution flows into cycle tank before pump and is added Na2CO3; then it is injected by pump into desulfurization tower to remove SO2 for cycle use. Desulfurization product is finally oxidized to be gypsum slurry CaSO4.
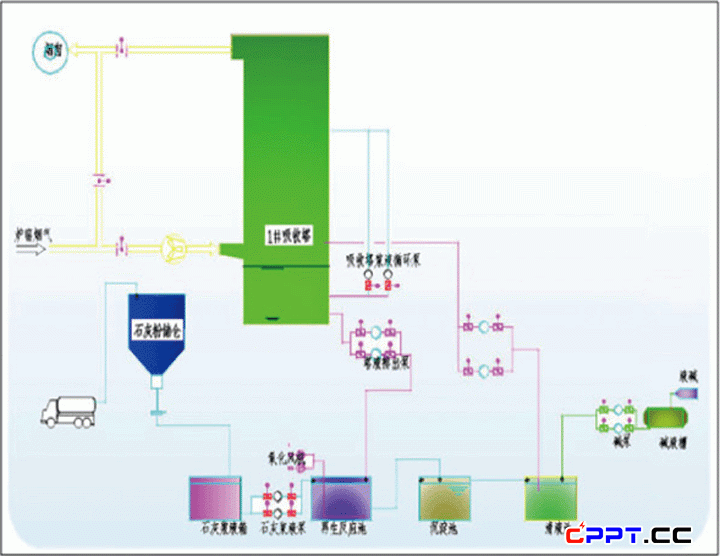
二、工艺流程图 Process Chart

三、性能保证
1、脱硫效率≥90%。
2、脱硫系统阻力≤800Pa。
3、循环液再生反应充分。
4、脱硫后烟气中的残余水分≤75mg/Nm3
III. Process chartII. Process chart
1、Desulfurization rate ≥90%
2、Desulfurization resistance ≤800Pa
3、Full generative reaction of cycle solution
4、Residual moisture in flue gas after desulfurization ≤75mg/Nm
四、反应机理
IV. Reaction mechanismSO2
吸收反应:Na2CO3 + SO2 → Na2SO3 + CO2↑
2NaOH + SO2 → Na2SO3 +H2O
Na2SO3 + SO2 + H2O → 2NaHSO3
吸收剂再生反应(Generative reaction of absorbent):CaO+H2O→Ca(OH)2
Ca(OH)2 + Na2SO3 → 2NaOH + CaSO3